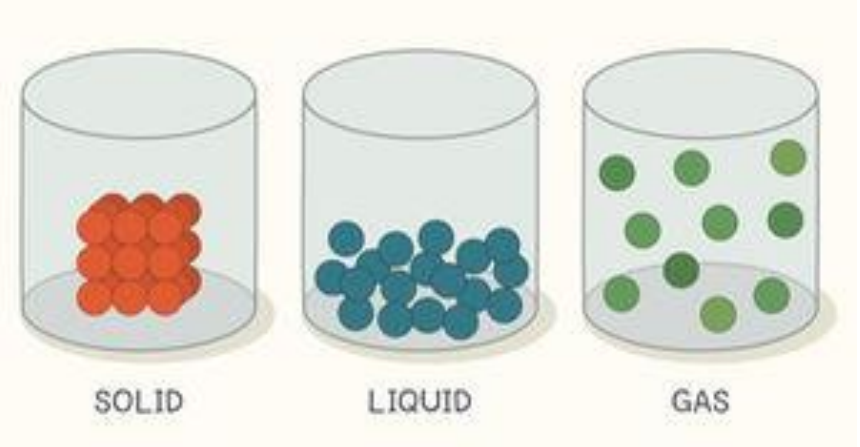
In a solid, molecules are packed together, and it keeps its shape. Liquids take the shape of the container. Gases spread out to fill the container.
Solid is one of the three main states of matter, along with liquid and gas. Matter is the "stuff" of the universe, the atoms, molecules and ions that make up all physical substances. In a solid, these particles are packed closely together and are not free to move about within the substance. Molecular motion for the particles in a solid is confined to very small vibrations of the atoms around their fixed positions; therefore, solids have a fixed shape that is difficult to change. Solids also have a definite volume; that is, they keep their size no matter how you try to change them
There are thousands of materials available for use in engineering applications. Most materials fall into one of three classes that are based on the atomic bonding forces of a particular material. These three classifications are metallic, ceramic and polymeric. Additionally, different materials can be combined to create a composite material.
Matters is defined as any substance that has mass and occupies space
Matter:
• atoms
• molecules
• ions
Atoms are the smallest unit of matter that can't be broken down chemically. Molecules are groups of two or more atoms that are chemically bonded. Ions are atoms or molecules that have gained or lost one or more of their valence electrons and therefore have a net positive or negative charge.
An atom can be an ion, but not all ions are atoms. There are distinct differences between an atom and an ion.